论文总字数:18599字
目 录
摘要 3
ABSTRACT 4
第一章 绪论 6
1.1 研究背景和意义 6
1.2 国内外研究的进展 6
第二章 实验与研究方法 8
2.1 观测地点介绍 8
2.2 仪器介绍 9
2.2.1水溶性离子检测仪 9
2.2.2 采样仪介绍 9
第三章 南京地区秋冬季气溶胶化学组分分析及来源分析 10
3.1 实验实施及方法 10
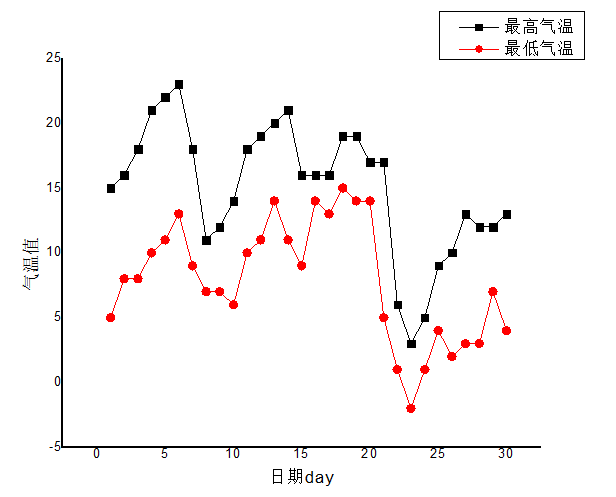
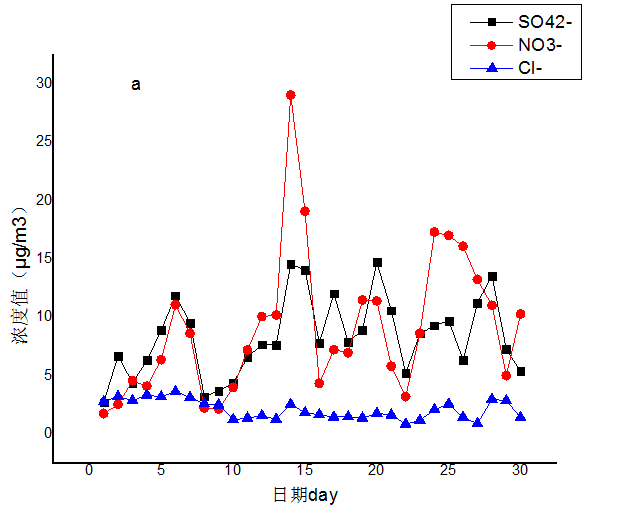
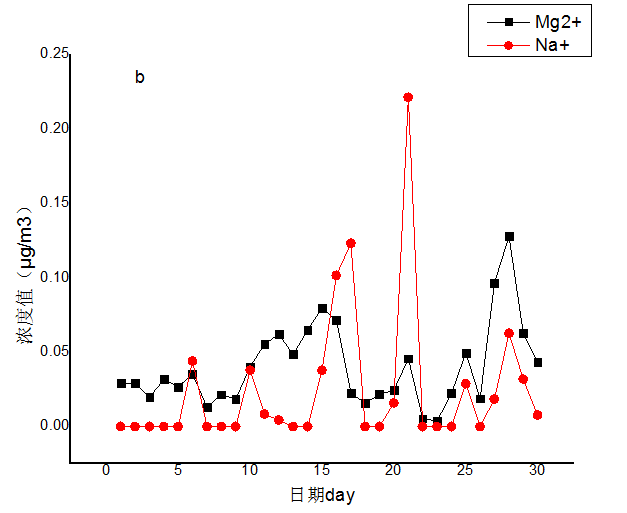
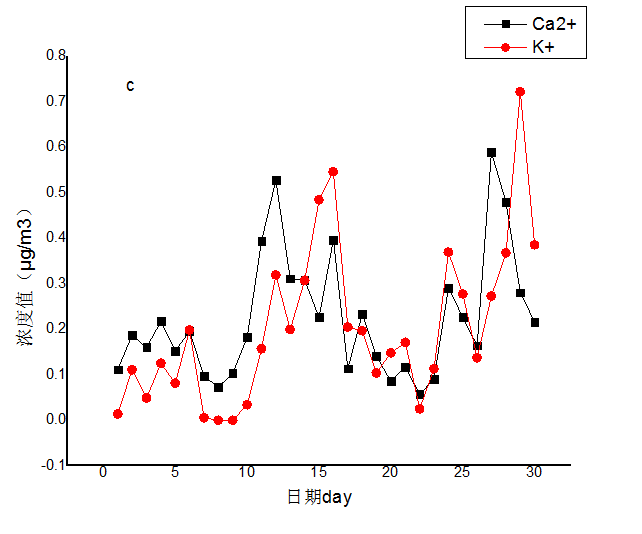
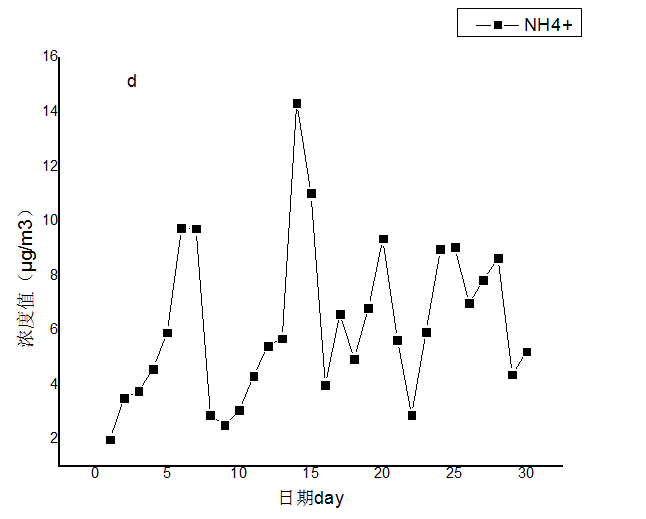
3.2 水溶性离子月变化特征分析 10
3.3霾天与非霾天的比较 14
3.4 结论 18
致谢 19
参考文献 20
摘要
水溶性离子是大气气溶胶的重要组分之一,尤其重污染天浓度较高,它对于空气质量、能见度和气候变化都有重要的作用,甚至对人类身体健康、生活质量、工业生产影响很大。近年来,南京地区经济迅速发展,机动车数量迅速攀升,各种工业生产增加,虽然南京市作出了一系列相应环保措施,但空气污染事件仍时有发生,而秋冬季依然是雾霾频发期。
本论文通过对大气气溶胶采样分析,并结合气溶胶前始气体和气象观测数据,研究了秋冬季南京地区重污染天气溶胶粒子化学组分,理解气溶胶化学组分与空气质量、能见度的联系,理解南京地区秋冬季污染的形成机制与原因,以此为南京地区空气质量的改善提供理论依据。
根据2016年南京地区秋冬季重污染天气溶胶水溶性离子化学成分的观测数据发现,秋冬季霾事件的发生次数明显多于夏季,导致现象频繁发生的一个重要原因是硝酸根离子、硫酸根离子的浓度大幅度升高。综合来看,空气质量变差的过程往往伴随着风速较小(一般小于 1m/s),累积降水量相对偏少等过程 ,降水和风力的增加有利于污染物浓度降低。SO42-、NH4 、NO3-浓度变化特征基本趋势一致,具有良好的对应关系,说明二者可能受相同因素的影响。南京冬季(12月份)观测期间的SO42-、NO3-和 NH4 是主要的水溶性离子。且NO3-gt;SO42-gt;NH4 。 霾天的二次离子的富集除物理因素外还有化学因素。 霾天 SO42- 和 NO3- 的浓度均比非霾天有所增加,尤其NO3-上升幅度更大,表明霾天更有利于 SO2和 NOX向SO42- 和 NO3-的转化,其中NOX的氧化最为显著。霾期间移动污染源的相对贡献率高于非霾期。霾天SO42-早上和夜间浓度较高,表明机动车排放源的重要影响,且交通排放对霾天 NO3-污染有重要贡献;除一次前体物外,二次化学反应对SO42-和 NO3-浓度变化的影响极大,随着光化学反应的强烈程度变化显著。NO3-在上午(8:00~9:30) 和夜间浓度相对较高,是因为边界层高度的变化和夜间低温高湿的天气条件有利于发生均相反应和液相反应。SO42-浓度的增加主要是来自于光化学反应,其总体日变化趋势与NO3-日变化趋势基本一致。
关键词:重污染天,水溶性离子。
ABSTRACT
The water soluble ions is an important component of the atmospheric aerosol,especially in the heavy pollution days its concentration become higher.It plays an important role in air quality,visibility and climate change and even has a great impact on human health,quality of life and industrial production.In recent years,with the rapid economic development in Nanjing,the number of motor vehicles has risen rapidly,and various industrial productions have increased.Although Nanjing has made a series of corresponding environmental protection measures,but air pollution incidents still occur,and autumn and winter are still frequent periods of haze.
This paper based on atmospheric aerosol sampling and analysis,and combined with the initial gas of aerosol and meteorological observation data,then it studied aerosol particle chemical composition of heavy pollution weather and the reason of pollution in autumn and winter of Nanjing ,so as to provide theoretical basis for the improvement of air quality in Nanjing region.
According to the chemical constituents observation data of water soluble ions in autumn and winter season of Nanjing area ,2016,it can be obtained that the occurrence of haze events in autumn and winter is obviously more than that in summer,an important cause of the frequent occurrence is that the concentration of nitrate ion and sulfate ion is substantially increase.Generally speaking,the process of deterioration of air quality is often accompanied by such processes as less wind speed(generally less than 1m/s) and relatively small amount of accumulated precipitation.The increase of precipitation and wind power are beneficial to the decrease of pollutant concentration.The basic characteristics of SO42-,NH4 and NO3- concentration changes are consistent,and they have good correspondence.This shows that the two factors may be affected by the same factors.SO42-,NH4 and NO3- are the major water soluble ions during the winter (December) observation in Nanjing,and NO3-gt;SO42-gt;NH4 .In addition to the physical factors,there are chemical factors in the enrichment of the two ion in the haze days.In haze days,the concentrations of NO3- and SO42- were increased in non haze days,especially NO3- .The results showed that haze days were more favorable for the transformation of SO2 and NOX to SO42- and NO3-,the oxidation of NOX is most significant among them.During the haze period,the relative contribution rate of mobile pollution sources is higher than non haze period.Haze days’ SO42- is higher in the morning and nighttime,indicating an important impact of vehicle emissions sources,and traffic emissions have important contribution to NO3- in haze days.In addition to the precursor,the two chemical reaction has a great influence on the concentration SO42- and NO3-,and varies greatly with the degree of photochemical reaction.The concentration of NO3- in the morning(8:00~9:30) and nighttime is relatively high because of the change of boundary layer height and low temperature and high humidity weather conditions in nighttime,which is beneficial to homogeneous reaction and liquid phase reaction.The increase of SO42- mainly comes from the phitochemical reaction ,and the overall diurnal variation trend is basically consistent with that of NO3-.
Key words:haze days,water soluble ions.
剩余内容已隐藏,请支付后下载全文,论文总字数:18599字
相关图片展示: